High throughput newborn screening for aromatic L-amino acid decarboxylase deficiency by analysis of concentrations of 3-O-methyldopa from dried blood spots
Brennenstuhl H, et al. J Inherit Metab Dis. 2020;43(3):602–610.
Publication Date | May 2020 (epub 6 Jan 2020)
Authors | Brennenstuhl H, Kohlmüller D, Gramer G, Garbade SF, Syrbe S, Feyh P, Kölker S, Okun JG, Hoffmann GF, Opladen T.
Citation | J Inherit Metab Dis. 2020;43(3):602–610.
https://pubmed.ncbi.nlm.nih.gov/31849064/
Researchers in Heidelberg, Germany, have developed a novel high-throughput method for the detection and measurement of 3-O-methyldopa (3-OMD) in dried blood spots (DBS). It represents a non-invasive, simple, rapid and valid method for diagnosing aromatic L-amino acid decarboxylase (AADC) deficiency in newborn screening (NBS).1
AADC deficiency is a rare autosomal recessive disorder of biogenic amine metabolism. AADC catalyses levodopa (L-Dopa) to dopamine, 5-hydroxytryptophan (HTP) to serotonin, and L-tryptophan to tryptamine. Genetic variants of the AADC-coding DDC gene result in reduced synthesis of catecholamines and serotonin. In AADC-deficient patients, the phenotypic spectrum ranges from mild courses with predominantly autonomous symptoms to severe cases with early-onset muscular hypotonia, movement disorders, and developmental delay.
Positive treatment outcomes are correlated with early, pre-symptomatic diagnosis and treatment initiation, emphasising the need for early and reliable diagnostic approaches, e.g. in NBS.2,3 The characteristic cerebrospinal fluid (CSF) profile reveals low concentrations of the dopamine and serotonin degradation products homovanillic acid (HVA) and 5-hydroxyindoleacetic acid (5-HIAA), and elevated concentrations of L-Dopa and 3-OMD. 3-OMD accumulates in AADC-deficient patients due to the inability to utilise L-Dopa generated by tyrosine hydroxylase. Its concentration is elevated in dried blood spots of AADC-deficient patients and is therefore a candidate biomarker for NBS.
Measurement of 3-OMD in DBS using LC-MS/MS was proposed in 2016 as a suitable method to identify AADC-deficient patients pre-symptomatically in the newborn period.4 The novel approach proposed by Brennenstuhl, et al. eliminates the use of column separation, thereby shortening the measurement time per sample. They established a tandem mass spectrometry method to quantify 3-OMD in DBS and successfully tested it in 38,888 unaffected newborns, 14 heterozygous DDC variant carriers, 7 known AADC-deficient patients, and 1,079 healthy control subjects.1
The group obtained 38,888 newborn DBS samples after parental consent during regular NBS, and DBS filter cards from 7 confirmed AADC-deficient patients between the age of 1 and 28 years (n = 2 male, n = 5 female). To rule out that heterozygous variant carriers were detected, 14 DBS samples were obtained from confirmed carriers of monoallelic genetic variants of the DDC gene. No clinical symptoms which could be related to AADC deficiency were reported in any of these subjects. Cut-off values were adjusted according to age (5 µmol/l for NBS, 3 µmol/l for children ranging from 28 days to 10 years, and 2 µmol/l for children 10 to 18 years).1
3-OMD concentrations in the healthy newborns revealed a mean of 1.16 µmol/l (SD = 0.31; range 0.31–4.6 µmol/l). Non-AADC control subjects showed a mean 3-OMD concentration of 0.78 µmol/l (SD = 0.34; range 0.24–2.36 µmol/l) with a negative correlation with age. The highest concentration of 3-OMD was found in the NBS filter card of a confirmed AADC-deficient patient, with a mean 3-OMD of 35.95 µmol/l (SD = 0.77 µmol/l). The 3-OMD measurement in the DDC variant carriers showed a mean concentration of 0.69 µmol/l with an SD of 0.19 µmol/l (Figure 1).1
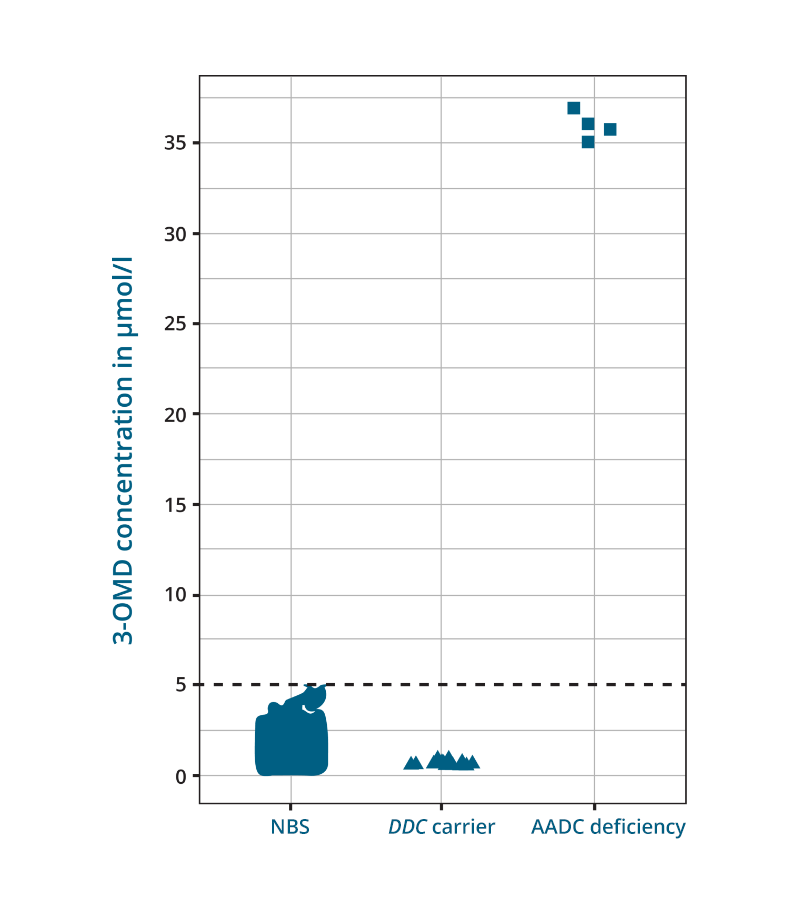
Adapted from: Brennenstuhl H, et al. J Inherit Metab Dis. 2020;43:602–610.
Figure 1: 3‐OMD concentration in DBS samples of 38,888 newborns, DBS samples of 14 adult carriers and 1 NBS sample of an AADC deficiency patient. Measurement of 3‐OMD in non‐AADC deficiency newborns, carriers of monoallelic DDC variants, and one NBS DBS sample of a patient with AADC deficiency. The patient revealed a mean 3‐OMD concentration of 35.95 μmol/L; displayed are four independent measurements of the same DBS filter card.
The diagnostic gap between initial symptoms and the confirmed diagnosis remains large for rare neurotransmitter related disorders such as AADC deficiency, where clinical symptoms are unspecific or diagnostic procedures are invasive and limited to highly specialised laboratories. A valid, stable, and reliable method to identify newborns suffering from AADC deficiency is necessary for pre-symptomatic diagnosis and early treatment initiation. Brennenstuhl, et al. propose their novel high-throughput method to measure 3-OMD concentrations in DBS be integrated into existing NBS programs and included in the diagnostic workflow to investigate unexplained movement disorders, developmental delay or intellectual disability in patients aged 0–18 years, enabling early diagnosis of AADC deficiency. In case of an elevated DBS 3-OMD concentration, confirmatory diagnostics using an enzymatic assay, or genetic testing is recommended.1
- Brennenstuhl H, et al. J Inherit Metab Dis. 2020;43(3):602–610.
- Kojima KT, et al. Brain. 2019;142(2):322–333.
- Tseng CH, et al. Ann Neurol. 2019;85(5):644–652.
- Chien YH, et al. Mol Genet Metab. 2016;118(4):259–263.
